MediumBank adheres to the quality policy of "Towards excellence, quality oriented; steady and far-reaching, help the world to secure the state"; through continuous optimisation of the quality management system and benchmarking with the latest international quality management methods, MediumBank continues to provide customers with effective and quality-controlled upstream products and services in biopharmaceuticals.
MediumBank has set up a perfect and continuously optimised quality management system for culture media with reference to ISO 13485:2016 and GMP, and the system has got ISO 13485 certification by TUV.Using the quality management concept centred on risk management, the system identifies risks throughout the entire life cycle of cell culture media and the entire process to ensure that the products supplied to customers are stable. In order to satisfy customers' requirements for simultaneous declaration at domestic and internation, MediumBank has carried out the filing of DMF for culture media, which can support customers' IND and NDA declarations.
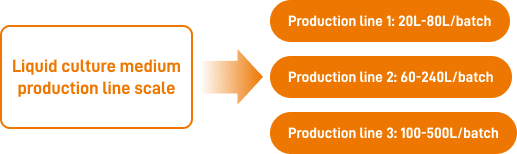
MFG1 capacity: 10 million liters/year, in line with GMP level put into production and has passed ISO13485 quality system certification.
1. For the grinding process of dry powder culture medium, it adopts needle mill imported from Germany, which can guarantee the homogeneity of dry powder particle size and realize temperature control in the process of grinding;
2. For the mixing process of dry powder culture medium, it first pre-treats the trace substances and then adopts two-dimensional mixing, which can guarantee the homogeneity of the mixing of the whole process.

1. Strict control of dissolving temperature and pH during the production of liquid culture medium to ensure the quality of liquid culture medium;
2. Strict microorganism control during the production process.